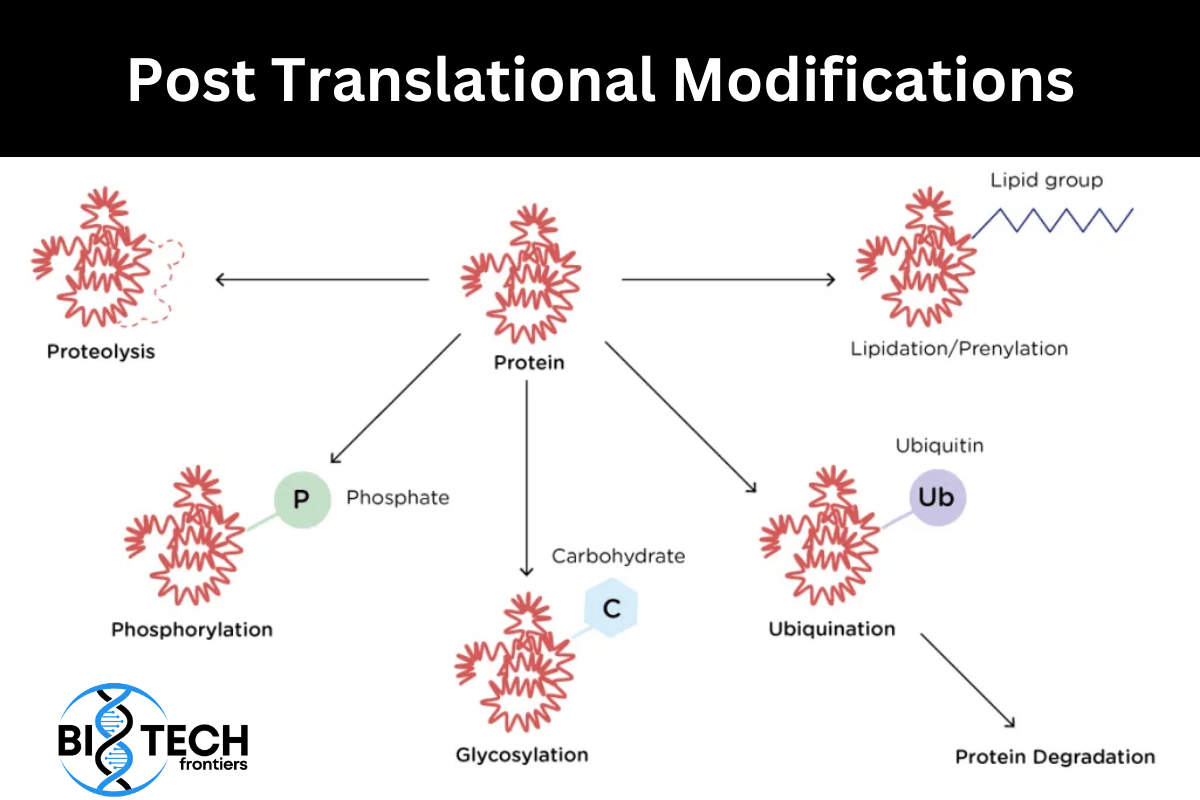
Many newly synthesized proteins (bacterial, archaeal, and eukaryotic) undergo various types of changes prior to their 3D folding into final biologically active conformation. These changes are know as post translational modification. Some common post translational modifications are:
Contents
- Amino-Terminal and Carboxyl-Terminal Modifications
- Loss of Signal Sequences
- Modification of Individual Amino Acids
- Attachment of Carbohydrate Side Chains
- Addition of Isoprenyl Groups
- Addition of Prosthetic Groups
- Proteolytic Processing
- Formation of Disulfide Cross-Links
Amino-Terminal and Carboxyl-Terminal Modifications
- Removal of N-formylmethionine (in bacteria) or methionine (in eukaryotes)
- In some cases, carboxyl-terminal) residues may be removed enzymatically in formation of the final functional protein.
- In as many as 50% of eukaryotic proteins, the amino group of the amino-terminal residue is N-acetylated after translation.
Loss of Signal Sequences
- 15 to 30 residues at the amino-terminal end of some proteins play a role in directing the protein to its ultimate destination in the cell.
- Such signal sequences are eventually removed by specific peptidases.
Modification of Individual Amino Acids
- The hydroxyl groups of certain Ser, Thr, and Tyr residues of some proteins are enzymatically phosphorylated by ATP eg casein protein.
- In many cases, phosphorylation-dephosphorylation cycles regulate the activity of many enzymes and regulatory proteins.
- Extra carboxyl groups may be added to Glu residues of some proteins. Eg. the blood-clotting protein prothrombin contains a number of –carboxyglutamate residues in its amino-terminal region, introduced by an enzyme that requires vitamin K. These carboxyl groups bind Ca+2, which is required to initiate the clotting mechanism.
- Monomethyl- and dimethyllysine residues occur in some muscle proteins and in cytochrome c.
- The calmodulin of most species contains one trimethyllysine residue at a specific position. In other proteins, the carboxyl groups of some Glu residues undergo methylation, removing their negative charge.

Attachment of Carbohydrate Side Chains
- The carbohydrate side chains of glycoproteins are attached covalently during or after the synthesis of polypeptide.
- In some glycoproteins, the carbohydrate side chain is attached enzymatically to Asn residues (N-linked oligosaccharides), in others to Ser or Thr residues (O-linked oligosaccharides).
- Many proteins that function extracellularly (such as serum proteins), as well as the lubricating proteoglycans that coat mucous membranes, contain oligosaccharide side chains.

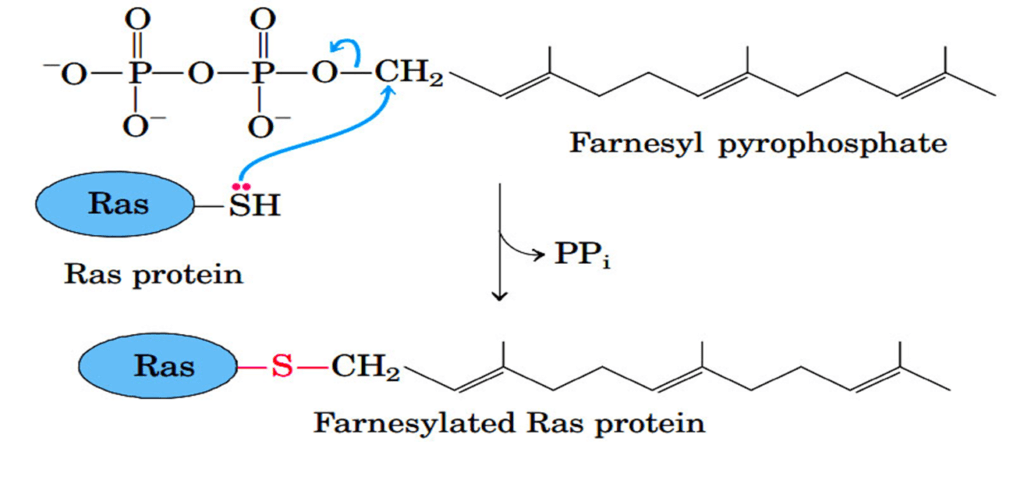
Addition of Isoprenyl Groups
- Some eukaryotic proteins are modified by the addition of isoprenyl groups.
- A thioether bond is formed between the isoprenyl group and a Cys residue of the protein.
- The isoprenyl groups are derived from pyrophosphorylated intermediates of the cholesterol biosynthetic pathway, such as farnesyl pyrophosphate.
- Examples include Ras proteins (product of ras proto-oncogenes), G proteins, and lamins (proteins of nuclear matrix).
- The isoprenyl group helps to anchor the protein in a membrane.
- The transforming (carcinogenic) activity of the ras oncogene is lost when isoprenylation of the Ras protein is blocked, a finding that has stimulated interest in identifying inhibitors of this posttranslational modification pathway for use in cancer chemotherapy.
Addition of Prosthetic Groups
- Covalently bound prosthetic groups are needed by many proteins for their activities such as biotin molecule of acetyl- CoA carboxylase and the heme group of hemoglobin or cytochrome c.
Proteolytic Processing
- Many proteins are initially synthesized as large, inactive precursor polypeptides that are proteolytically trimmed to form their smaller, active forms.
- Examples include proinsulin, some viral proteins, proteases such as chymotrypsinogen and trypsinogen, blood clotting factors, caspases etc
Formation of Disulfide Cross-Links
- After folding into their native conformations, some proteins form intrachain or interchain disulfide bridges between Cys residues.
- In eukaryotes, disulfide bonds are common in proteins to be exported from cells.
- The disulfide bonds help to protect the native conformation of the protein molecule from denaturation in the extracellular oxidizing environment.
Protein degradation
- Abnormal or unwanted proteins undergo degradation in order to recycle the amino acid and prevent their accumulation.
- The half-lives of eukaryotic proteins vary from 30 seconds to many days.
- Rapidly degraded proteins include defective proteins (incorrectly inserted amino acids or damaged proteins), regulatory metabolic proteins and proteins with short half-lives.
- These proteins generally degraded in both bacterial and eukaryotic cells by selective ATP-dependent cytosolic systems.
- Another protein degradation system is existed in the lysosome which recycles the amino acids of membrane proteins, extracellular proteins, and proteins with characteristically long half-lives.
- In E. coli, many proteins are degraded by an ATP-dependent protease called Lon.
- The Lon is activated in the presence of defective proteins or those slated for rapid turnover; two ATP molecules are hydrolyzed for every peptide bond cleaved.
vThe precise role of this ATP hydrolysis is not yet clear. Once a protein has been reduced to small inactive peptides, other ATP-independent proteases complete the degradation process.
Ubiquitin dependent protein degradation
- Ubiquitin (76 amino acid residues) is a highly conserved protein used to mark the proteins for degradation in all the eukaryotic cells including humans.
- Ubiquitin is covalently linked to proteins destined for degradation via an ATP-dependent pathway involving three separate enzymes (E1, E2, and E3).
- Ubiquitinated proteins are degraded by a large complex known as the 26S proteasome (Mr 2.5 x106).
- The proteasome consists of two copies each of at least 32 different subunits, most of which are highly conserved from yeasts to humans.
- The 20S core particle consists of four rings; the outer rings are formed from seven subunits, and the inner rings from seven subunits. Three of the seven subunits in each ring have protease activities, each with different substrate specificities.
- The stacked rings of the core particle form the barrel-like structure within which target proteins are degraded.
- The 26S proteasome can be effectively “accessorized,” with regulatory complexes changing with changing cellular conditions.
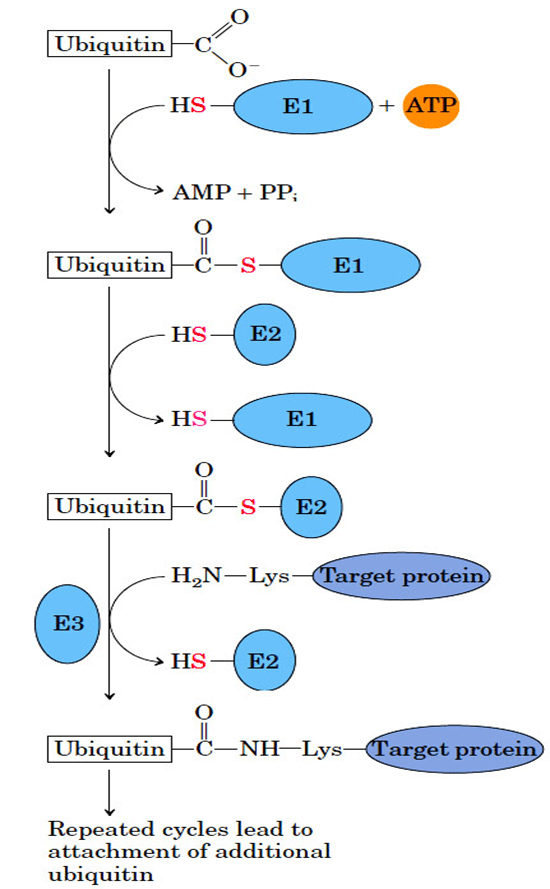
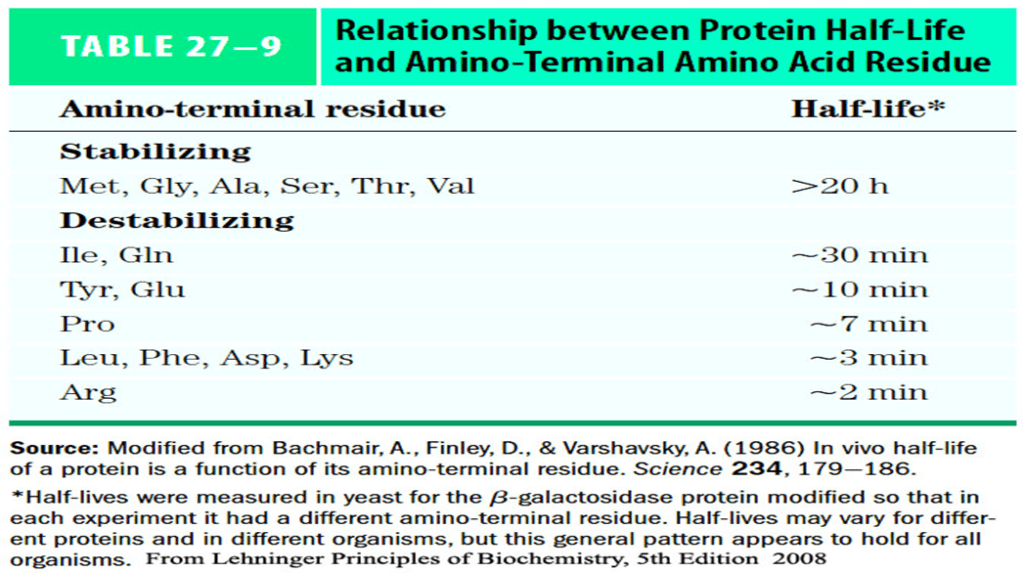
- Signals that trigger ubiquitination are not yet defined however, one simple signal i.e., the amino terminus residues has profound effect on ubiquitination.
- These amino-terminal signals have been conserved over billions of years of evolution and are the same in bacterial protein degradation systems and in the human ubiquitination pathway.
- Ubiquitin-dependent proteolysis is as important for the regulation of cellular processes as for the elimination of defective proteins.
- Ubiquitin-dependent destruction of cyclin is critical to cell-cycle regulation.
- An inability to degrade certain proteins that activate cell division (the products of oncogenes) can lead to tumor formation, whereas a too-rapid degradation of proteins that act as tumor suppressors can have the same effect.
- The ineffective or overly rapid degradation of cellular proteins also causes various pathological conditions such as renal diseases, asthma, neurodegenerative disorders such as Alzheimer’s and Parkinson’s diseases, cystic fibrosis (caused in some cases by a too-rapid degradation of a chloride ion channel, with resultant loss of function); Liddle’s syndrome (in which a sodium channel in the kidney is not degraded, leading to excessive Na absorption and earlyonset hypertension).
- Drugs designed to inhibit proteasome function are being developed as potential treatments for some of these conditions.
Non-ribosome protein synthesis
- Many bacteria and fungi also possess an alternative protein-synthesis pathway, which they use to synthesize a few short, specialized proteins.
- This protein synthetic pathway does not rely on the ribosome; instead, it uses enzyme nonribosomal peptide synthetase (NRPS)—a large protein complex for the peptide synthesis.
- NRPS not only uses 20 standard amino acid but also employ hundreds of different nonprotein amino acids.
- NRPS can synthesis linear as well as cyclic peptides.
- It is able to produce some proteins with unusual structures which often resist degradation and may go undetected by pathogens.
- Few examples included vancomycin, syringomycin, cyclosporin and vibriobactin etc

References
- Lehninger Principles of Biochemistry ( 5E 2008 ISBN 9780716771081 ) David L. Nelson, Michael M. Cox, W.H. Freeman and Company NY
- Genetics: A conceptual Approach by Benjamin A. Pierce, 3rd edition 2009, WH Freeman and Company
- Principles of Genetics Sixth Edition by D. Peter Snustad and Michael J. Simmons; John Wiley & Sons, Inc.
- Genes VIII 2004 by Benjamin Lewin Published by Pearson Prentice Hall Pearson Education, Inc.