DNA serves as genetic material due to self-replicating properties and repair mechanisms. DNA contains the genetic code that provides instructions for all cellular processes and development in an organism. Any changes or aberrations in the DNA can lead to abnormalities and disease states. These changes in DNA structure can occur at the chromosomal level or at the level of individual genes.
Chromosomal Aberrations
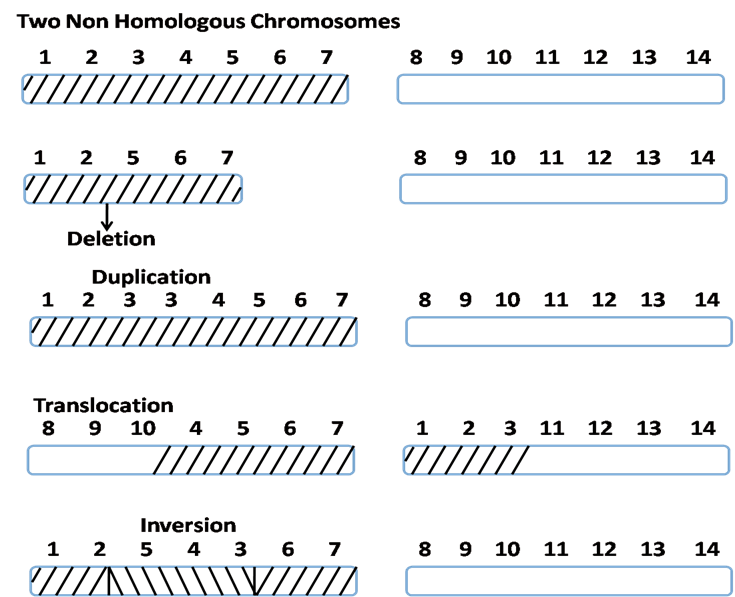
- Any change in normal chromosomes is termed chromosomal aberration, which may be structural or numerical.
- Structural changes include deletion, duplication, translocation, and inversion.
- Numerical changes involve aneuploidy (monosomy, trisomy) and euploidy (haploid, diploid).
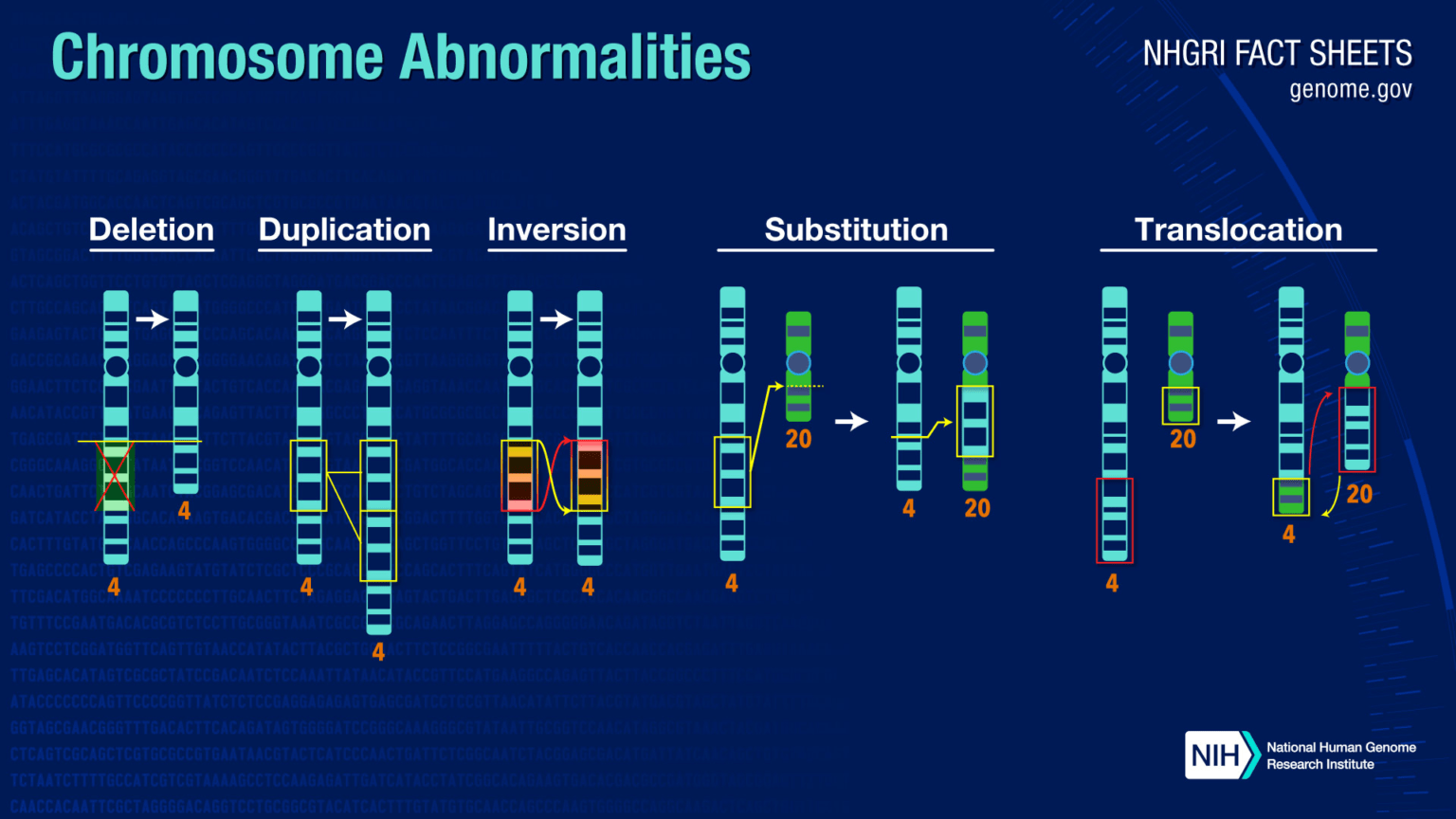
Structural Changes
1. Deletion – Loss of a chromosomal segment. Examples:
- Wolf-Hirschhorn syndrome: partial deletion of chromosome 4 short arm (4p-) (Schinzel, 2001)
- Jacobsen syndrome: deletion of chromosome 11 long arm terminus (11q-) (Grossfeld et al., 2004).
- Cri du chat syndrome: partial deletion of chromosome 5 short arm (5p-) (Zhang et al., 2005)
2. Duplication – Addition/copy of a chromosomal segment. Examples:
- Bar eye in Drosophila: duplication of 16A region on X chromosome (Lindsley & Zimm, 1992).
- Charcot-Marie-Tooth disease: duplication of 17p12 region (Patel & Lupski, 1994).
3. Translocation – Movement of a chromosomal segment to a different chromosome. Can be reciprocal or Robertsonian. Implicated in leukemias and Ewing’s sarcoma (Arvand & Denny, 2001; Turc-Carel et al., 1988).
4. Inversion – Reversed orientation of a chromosomal segment. Para- or pericentric. Can result in position effects on gene expression (Kirkpatrick, 2010).
Numerical Changes:
The numerical changes are mainly of two types.
Aneuploidy
Change (increase or decrease) of one or two chromosome from a chromosome pair.
- Monosomy (2n-1): Turner syndrome (XO): Loss of one X chromosome.
- Nullisomy (2n-2): Loss of one complete set of chromosomes (patient will not survive).
- Trisomy (2n+1)
- Down syndrome or Mongolism: Trisomy of 21st pair of chromosomes
- Edwards syndrome: Trisomy of18th pair of chromosomes
- Patau syndrome: Trisomy of 13st pair of chromosomes.
- Klinefelter syndrome (47, XXY): Extra X chromosome in male sex chromosome set.
- Tetrasomy (2n+2): 48(XXXX): Two extra X chromosome.
Euploidy
Change (increase or decrease) of complete set of chromosomes.
- Haploid (n): Have single set of chromosomes.
- Diploid (2n): Have two set of chromosomes.
Gene Mutations and Genetic Disorders
Specific gene defects can lead to inborn errors of metabolism, loss of protein function, toxic metabolite accumulation and disease:
- Cystic fibrosis: Mutation in CFTR gene encoding chloride ion transporter causes altered ion and water transport in lungs and other organs (Quinton, 1999). It is caused A mutation CFTR gene due to deletion of three nucleotides, results loss of one amino acid (phenylalanine) at 508th position in the protein.
- Duchenne muscular dystrophy: Mutation in dystrophin gene disrupts muscle fiber stability and causes progressive muscle wasting (Hoffman et al., 1987)
- Sickle cell anemia: Point mutation in beta globin chain causes hemoglobin polymerization and red cell sickling (Pauling et al., 1949).
- Hemophilia: Mutations in clotting factor genes F8 (hemophilia A) or F9 (hemophilia B) cause impaired blood clotting ability (Giannelli et al., 2018).
Several other examples like Pompe disease (acid maltase deficiency) (Hirschhorn & Reuser, 2001), Lesch-Nyhan syndrome (HPRT deficiency) (Torres & Puig, 2007), etc. highlight the dramatic phenotypes that can arise from single gene defects.
DNA Repair Defects and Cancer
- Nucleotide excision repair (NER) pathway fixes bulky DNA lesions. Mutations affecting NER genes can cause cancer-prone syndromes like xeroderma pigmentosum (XP) (Cleaver et al., 2009).
- Faulty tumor suppressor genes (e.g. RB, p53) or hyperactivation of proto-oncogenes (e.g. RAS, MYC) via mutation can trigger unchecked cell proliferation and tumor formation (Vogelstein & Kinzler, 2004).
- Chromosomal translocations are common in leukemias and lymphomas, juxtaposing proto-oncogenes next to highly active promoters (e.g. BCR-ABL in CML) (Nowell & Hungerford, 1960; Rowley, 1973).
Purine and Pyrimidine Metabolism Related Disorders
- Lesch-Nyhan Syndrome:
- X-linked recessive disorder caused by HPRT deficiency, leading to hyperuricemia and gout.
- Adenine Phosphoribosyltransferase Deficiency (APRT):
- Autosomal recessive disorder causing adenine accumulation and urinary tract issues.
- Phosphoribosylpyrophosphate Synthetase Superactivity:
- X-linked recessive disorder causing purine overproduction, leading to hyperuricemia, gout, and developmental abnormalities.
- Adenosine Deaminase Deficiency:
- Causes severe combined immunodeficiency (SCID) due to impaired DNA replication.
- Uridine Monophosphate Synthase Deficiency:
- Autosomal recessive disorder causing orotic acid accumulation, leading to anemia and other complications.
A wide spectrum of hereditary disorders and acquired diseases like cancer have now been traced to underlying abnormalities in DNA sequence, structure or regulation. Understanding the molecular genetic basis provides insight into pathological mechanisms and opens new avenues for predictive diagnosis and therapy.
References
- Arvand A, Denny CT. Biology of EWS/ETS fusions in Ewing’s family tumors. Oncogene. 2001;20(40):5747-5754.
- Cleaver JE, Lam ET, Revet I. Disorders of nucleotide excision repair: the genetic and molecular basis of heterogeneity. Nat Rev Genet. 2009;10(11):756-768.
- Giannelli F, Green PM. The molecular basis of haemophilia A and B. Baillieres Clin Haematol. 1998;11(2):211-228.
- Grossfeld PD, Mattina T, Lai Z, et al. The 11q terminal deletion disorder: a prospective study of 110 cases. Am J Med Genet A. 2004;129A(1):51-61.
- Hirschhorn R, Reuser AJJ. Glycogen Storage Disease Type II; Acid α-Glucosidase (Acid Maltase) Deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The Metabolic and Molecular Bases of Inherited Disease. 8th ed. New York, NY: McGraw-Hill; 2001.
- Hoffman EP, Brown RH, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51(6):919-928.
- Kirkpatrick DT. Position effects on gene expression. Curr Opin Cell Biol. 2010;22(3):365-369.
- Lindsley DL, Zimm GG. The Genome of Drosophila Melanogaster. Academic Press; 1992.
- Nowell PC, Hungerford DA. A minute chromosome in human chronic granulocytic leukemia. Science. 1960;132(3438):1497.
- Patel PI, Lupski JR. Charcot-Marie-Tooth disease: a new paradigm for the mechanism, diagnosis, and treatment of inherited disease. Trends Genet. 1994;10(12):429-438.
- Pauling L, Itano HA, et al. Sickle cell anemia, a molecular disease. Science. 1949;110(2865):543-548.